Blood Pressure Medication Recall List 2020
These batches were recalled from the pharmacies in Aruba because of. Find out which specific blood pressure medications are affected by the recall.
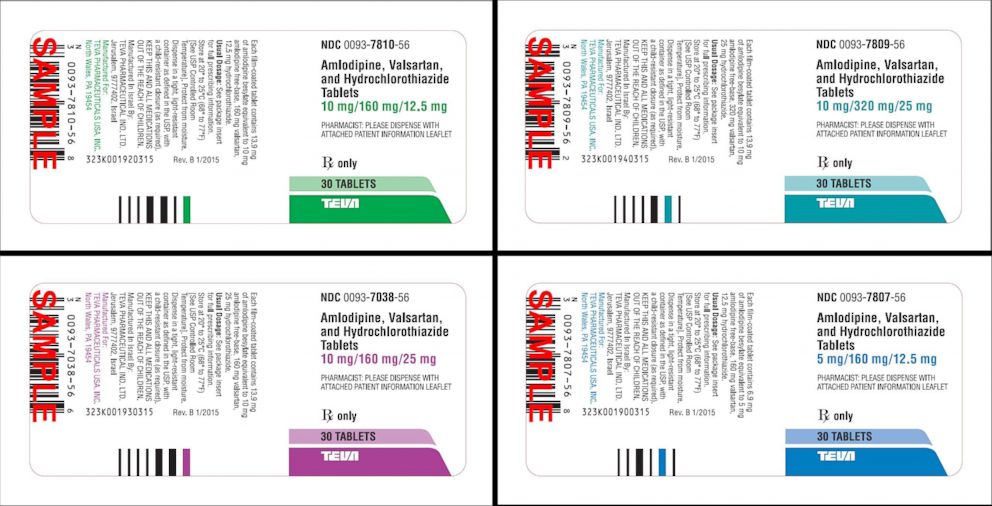
Blood Pressure Medication Recall What You Need To Know Abc News
The new recall brings the list of blood pressure medications recalled since October to 15.
Blood pressure medication recall list 2020. It is recorded while the blood pressure cuff is deflating. Drug Recall List Last Updated. Leave a Comment Lawsuits By Amy Gilmore.
Blood pressure medication recall expanded. October 2021 Drug Recall Details Contact Date Drug Recall Class Betaxolol Ophthalmic 01747870510 01747870511 Microbial Contamination of Sterile. Valsartan is a similar blood pressure medication to losartan.
In July 2018 valsartan was the first blood pressure drug recalled. Teva Pharmaceuticals has issued a voluntary recall. According to the FDA those recalls which began in June of 2018 involve a number of angiotensin II receptor blockers ARBs a type of medication used to treat high blood pressure.
The agency previously pushed recalls of the heartburn med Zantac and sartan blood pressure drugs for contamination with the same carcinogen. According to the FDA notice Patients who could be on a doubled dose of telmisartan for a prolonged period of time could experience low. Patients who are taking the recalled medication are advised to.
There is also the possibility that additional drugs will be added to the FDA list. The blood pressure medication was recalled after it was discovered that the bottles may contain 40-mg pills instead of 20-mg pills thus potentially jeopardizing the health and safety of those who take them. All have been found to have trace amounts of N-nitrosodiethylamine NDEA and are.
While youre on losartan may limit its effect on your blood pressure. A full list of Irbesartan and Hydrochlorothiazide tablets recalled can be viewed here. 1170 rows Search List of Recalled Angiotensin II Receptor Blockers ARBs including Valsartan Losartan and Irbesartan.
If the first number is above 130 or the second number is above 80 then a person is said to have high blood pressure. Below is a condensed version of the list of recalled ARB medications maintained by the Food and Drug Administration FDA. The recalled products have expiration dates ranging from October 2019 to July 2020.
The recall of Losartan is linked to a possible cancer-causing element known as NMBA. Most experts consider a normal blood pressure to be 12080 mm Hg. 34 rows CME America Recalls BodyGuard Infusion Pump System Due to Risk of Over- and Under.
The UK medicine. 1038 17 Jun 2021. Please see FDAgov DrugsDrug Safety and Availability Drug Recalls for additional information.
Losartan Recall Lawsuit 2021 Blood Pressure Drug Recalled. The FDA list includes dose lot. FDA Issues List of Safe Blood Pressure Meds.
Losartan Potassium Tablets USP 50mg 1000 count National Drug Code 13668-409-10. Dozens of medications used to treat high blood pressure including valsartan losatran and irbersartan have been recalled over the past several months as federal investigators discover. This is not a complete list of all recalls.
With unsafe levels of possible cancer-causing compounds are unaware that their health could be compromised due to their blood pressure medication. The recall was prompted by the discovery of cancer-causing impurities in the medications. April 5 2019 -- The FDA on Thursday issued a list of 40 blood pressure medicines it found free of contamination with the chemical nitrosamine an.
Between July 2018 and September 2019 the US. Ideally everybodys blood pressure should be below 13080 mm Hg. Cancer risks prompt FDA to recall more heartburn medication Fox Business via Yahoo Finance 2 years ago.
The newly recalled products can be identified by checking the product name manufacturer details and batch or lot number on the bottle containing these products. The Food and Drug. A Food and Drug Administration recall of a heart medication due to a cancer-causing chemical now includes two blood pressure medications.
1040 17 Jun 2021. Drug interactions such as dangerously low blood pressure may also occur if this prescription medicine is mixed with ACE inhibitors or aliskiren two other types of medications used to lower blood pressure DailyMed 2020. Click here for an updated list of Losartan products under recall.
Makers of blood-pressure medication expand their recall. COMMON blood pressure drugs have been recalled over contamination fears with a substance that can increase the risk of cancer. Health Canada has recalled some lots of blood pressure drugs under the names irbesartan losartan and valsartan as some may contain what Health.
Food and Drug Administration FDA announced the recall of hundreds of lots of blood pressure drugs. Blood pressure medication losartan recall. ORANJESTAD Aruba A drug importer notified the Health Inspectorate of Aruba that some batches of the drugs Losartankalium Valsartan and Irbesartan were recalled.
The Food and Drug Administration announced numerous recalls of heartburn and blood pressure. July 20 2021.

Blood Pressure Drug Recalled Over Risky Label Mix Up Everyday Health

More Blood Pressure Medication Recalls Due To Cancer Concerns
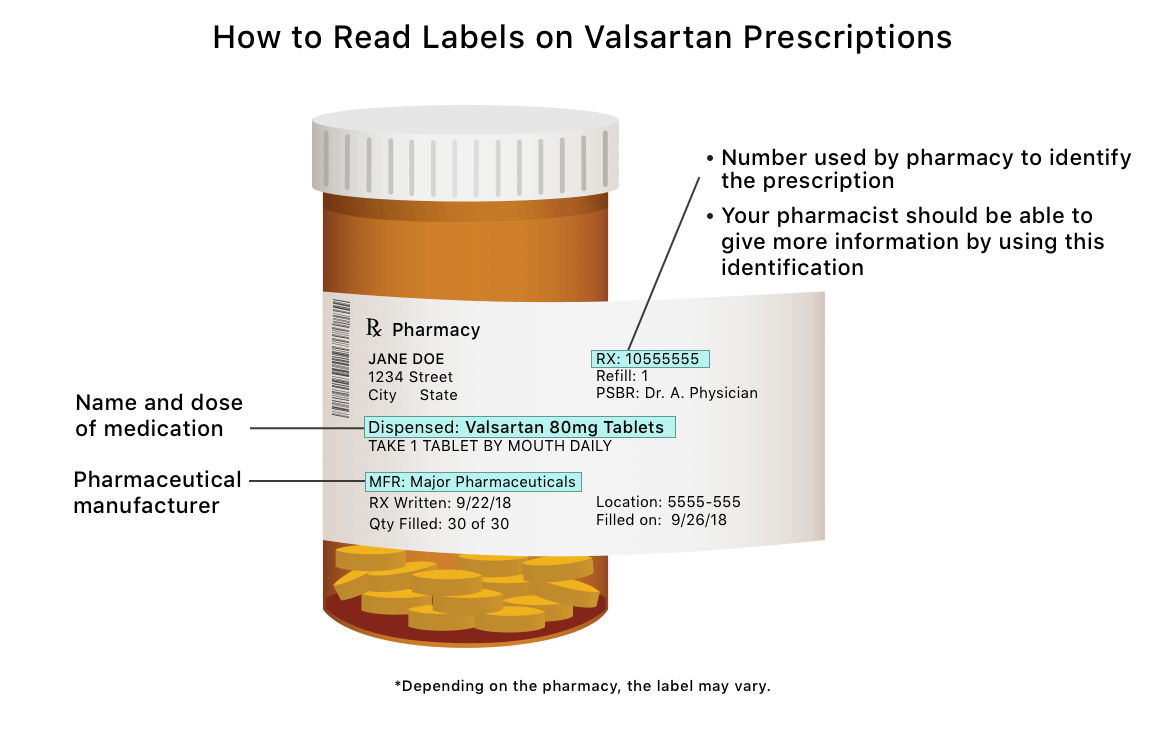
Valsartan Recall Why Is The Fda Recalling Valsartan
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Blood Pressure Medications Two To Avoid

Latest Blood Pressure Medication Recall List Updated September 2019 Oregonlive Com

New Year New Blood Pressure Medication Recall Drug Tablets Recalled Over Cancer Concern
Updated Common Blood Pressure Medications Containing Valsartan Recalled Over Impurity Concerns Self
Blood Pressure Medication Recall What You Need To Know Abc News

Fda Recalls Blood Pressure Drugs After Finding A Link To Cancer
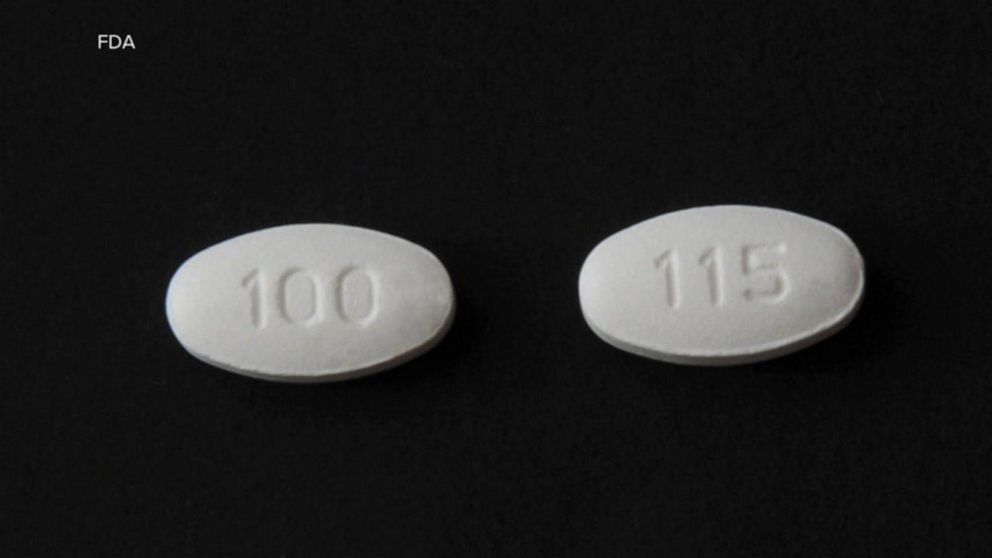
Blood Pressure Medication Recall What You Need To Know Abc News
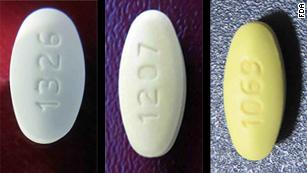
Fda Warns Of Common Blood Pressure Medicine Shortage Due To Recalls Cnn

Blood Pressure Drug Recall Losartan Valsartan Amlopidine Fortune

Blood Pressure Medication Recall Fda

Fda Recalls Blood Pressure Heart Failure Medications Due To Cancer Causing Agent Wset

Fda More High Blood Pressure Medications Recalled Due To Cancer Risk Wfla

Blood Pressure Medication Recall Chch

Blood Pressure Medications Recall Latest
/cloudfront-us-east-1.images.arcpublishing.com/gray/35NTEGPSZVLLDOMVOWDICBCC3U.jpg)
Is Your Blood Pressure Medication On The Recall List Find Out Here