Blood Pressure Medication Recall Atenolol
See below for a comprehensive list of adverse effects. Atenolol crosses the placental barrier and appears in cord blood.
Atenolol Tenormin Basics Side Effects Reviews
Chest pain angina.

Blood pressure medication recall atenolol. Auro Pharma Inc. A recall has been issued by Alembic Pharmaceuticals for one lot of. The recall of Pacific Atenolol 50mg tablets is due to a manufacturing problem.
Blood pressure medication recall. The usual dose is 50 - 100 mg once a day. Blood Pressure Medication Recall 2021.
Cardiac failure bradycardia dizziness fatigue and cold extremity. The usual dose is 50 - 100 mg once a day. The US Food and Drug Administration is recalling some medicines commonly used to help control blood pressure because batches of it may contain a chemical thats used to induce cancer in lab rats.
Last updated on Feb 21 2021. Commonly reported side effects of atenolol include. Dozens of blood pressure medications have been recalled since the first products were pulled off the shelf in July 2018 due to impurities.
The FDA continues to update the list of medications being recalled. The Ministry of Healths medicines regulatory arm Medsafe is working closely with the medicine supplier over the recall of all Pacific Atenolol Atenolol 50 mg and 100mg tablets which are used to treat various heart conditions including high blood pressure irregular heartbeats and to prevent angina chest pain. The usual dose is 50 mg - 100 mg once a day.
This includes some combination tablets which contain valsartan and amlodipine or valsartan amlodipine and hydrochlorothiazide. FDA has issued a recall of certain lots of angiotensin II receptor blocker ARB high blood pressure medication containing valsartan losartan or irbesartan. Joined Sandoz Canada Inc Sanis Health Inc Teva Canada Ltd Sivem Pharmaceuticals Inc sanofi-aventis Canada Inc and others in recalling certain products.
The usual dose is 100 mg once a day or 50 mg twice a day. What you need to know. The affected products all contained valsartan losartan.
High blood pressure hypertension. Lowering blood pressure may lower your risk of a stroke or heart attack. Our meta-analysis showed a significantly higher mortality 113 102-125 with atenolol treatment than with other active treatment in the five studies comprising 17671 patients who were followed up.
When atenolol was compared with other antihypertensives there were no major differences in blood pressure lowering between the treatment arms. The lot number can be found on the side of the. This template is a fully functional 5 page website with an examples page that gives examples of all the styles available with this design.
Food and Drug Administration FDA the recall affects all of the companys Irbesartan tablets 75 mg 150 mg and 300 mg and Irbesartan and Hydrochlorothiazide. September 24 2019 952 PM. Administration of atenolol starting in the second trimester of pregnancy has been associated with the birth of.
Hydrochlorothiazide and lisinopril is a combination medicine used to treat hypertension high blood pressure. Find out which specific blood pressure medications are affected by the recall Search List of Recalled Angiotensin II Receptor Blockers ARBs including Valsartan Losartan and Irbesartan. Atenolol is indicated for the treatment of hypertension to lower blood pressure.
Atenolol is used to treat various heart conditions including high blood pressure irregular heartbeats and to prevent angina chest pain. At 635 Vine St Winston Salem NC 27101. Atenolol Side Effects.
Atenolol Tenormin is a beta-blocker that affects the heart and circulation blood flow through arteries and veins. Irregular heart beat arrhythmia. Atenolol is used to treat angina chest pain and hypertension high blood pressure.
2 Ibesartan is widely used to treat blood pressure issues Credit. By Dawn Geske 032521 AT 1015 AM. Atenolol is also used to lower the risk of death after a heart attack.
Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events primarily strokes and myocardial infarctions. The Food and Drug Administration announced Wednesday that it will expand its recall of blood pressure medicines to include four lots of losartan after they were found to contain a cancer-causing. For reimbursement of the affected medication the recalled lots can be returned to Inmar Rx Solutions Inc.
Mylan New Zealand Ltd is recalling all Pacific Atenolol 50mg tablets dispensed to patients since 1 January 2010. The UK medicine regulator today issued a recall for 31 batches of products containing Irbesartan and two batches with Losartan in. Per a notice from the US.
Teva Pharmaceuticals has issued a voluntary recall of its amlodipinevalsartan combination tablets and amlodipinevalsartanhydrochlorothiazide combination tablets both used to treat high blood. Hypertension Drug Recalled Over Potential Death Risk. The researchers recorded both blood pressure variation as well as the blood pressure medication used by each participant.
Early stages of heart attack. Atenolol can cause fetal harm when administered to a pregnant woman.

Blood Pressure Medication Recalled For Label Mix Up Silive Com
Blood Pressure Medication Recall What You Need To Know Abc News

Blood Pressure Medications Two To Avoid

Blood Pressure Medication Recalled Because May Contain Stronger Dose Than Indicated Pennlive Com

Blood Pressure Medications Recall Latest
Atenolol National Backorder Diners Club
Why Is There A Recall On Atenolol Recall On Atenolol
Atenolol Tenormin Basics Side Effects Reviews

Atenolol Information Side Effects Warnings And Recalls

Mylan Recalled Three Blood Pressure Medications That Had Impurities

Atenolol Is A Medication Of The Beta Blockers Type Primarily Used To Treat High Blood Pressure And Angina Editorial Image Image Of Include Hospital 124342380
Blood Pressure Drug Recalled Over Potentially Life Threatening Label Mix Up Cbs News
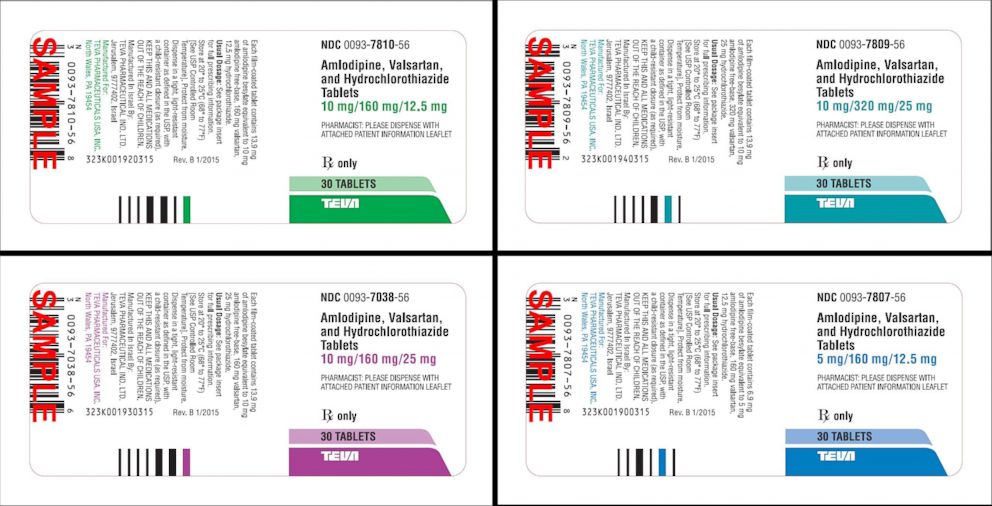
Blood Pressure Medication Recall What You Need To Know Abc News

Atenolol Information Side Effects Warnings And Recalls
Valsartan Losartan And Other Blood Pressure Medication Recalls 2018 19

Fda Announces Expanded Recall Of Widely Used Blood Pressure Drug
Recall Blood Pressure Medicine Lot Recalled Due To Label Mix Up Wtvc

